Any two charged objects will create a force on each other. Opposite charges will produce an attractive force while similar charges will produce a repulsive force. The greater the charges, the greater the force. The greater the distance between them, the smaller the force. For two spherically shaped charges the formula would look like:
  |
|
The forces are force pairs of each other so they will always be equal in size and opposite in direction.
COULOMB'S LAW IN VECTOR FORMSuppose that two charges,
Consider 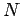 charges,
charges,  though
though  , which are located at position vectors
, which are located at position vectors 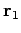 through
through 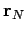 . Electrical forces obey what is known as the principle of superposition. The electrical force acting on a test charge
. Electrical forces obey what is known as the principle of superposition. The electrical force acting on a test charge 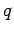 at position vector
at position vector 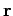 is simply the vector sum of all of the Coulomb law forces from each of the
is simply the vector sum of all of the Coulomb law forces from each of the 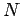 charges taken in isolation. In other words, the electrical force exerted by the
charges taken in isolation. In other words, the electrical force exerted by the 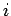 th charge (say) on the test charge is the same as if all the other charges were not there. Thus, the force acting on the test charge is given by
th charge (say) on the test charge is the same as if all the other charges were not there. Thus, the force acting on the test charge is given by
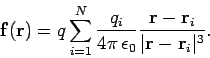 |
Example 4 Coulomb’s law for electrostatic force between two point charges and Newton’s law for gravitational force between two stationary point masses, both have inverse-square dependence on
the distance between the charges/masses. (a) Compare the strength of these forces by determining the ratio of their magnitudes (i) for an electron and a proton and (ii) for two protons. (b) Estimate the accelerations of electron and proton due to the electrical force of their mutual attraction .
ANS= USE F[e]/F[g]

No comments:
Post a Comment